- Thrombolytic
- Anti-inflammatory
- Cardioprotective effects


The mechanism of thrombolytic action is associated with direct destruction of fibrin, which form the basic framework of thrombus.
The mechanism of anti-inflammatory action is associated with effect on the oxidative function of blood neutrophils and tissue macrophages
Cardioprotective mechanism of action is associated with improved myocardial perfusion.

Indications for use: as an adjunct in the treatment of chronic venous insufficiency.
Contraindications for use: hypersensitivity to the drug, pregnancy, lactation, age 18 years (the efficacy and safety have not been established), peptic ulcer and duodenal ulcer (acute phase), co-administration with drugs containing divalent metal salts (calcium, magnesium, zinc, iron), and tetracycline antibiotics (tetracycline, chlortetracycline hydrochloride and oxytetracycline hydrochloride).
Dosing regimen, route of administration: The drug is used inside for 30 - 40 minutes before a meal to 800-1600 IU per day, divided into two doses. The maximum daily dose - 2000 units. The treatment course is 20 days. In the absence of efficiency throughout the course of treatment should consult a doctor. If necessary, may conduct repeated courses on the recommendation of a physician.
Precautions: polyvalent allergy, chronic obstructive pulmonary disease, the threat of bleeding from esophageal varices, urolithiasis, peptic ulcer and duodenal ulcer (in remission).
Symptoms of overdose, measures to aid in overdose: overdose was not observed.
Possible side effects: Allergic reactions, in rare cases, dyspeptic symptoms (nausea, vomiting, a feeling of heaviness in the stomach). Perhaps a temporary feeling of fullness in the lower extremities.

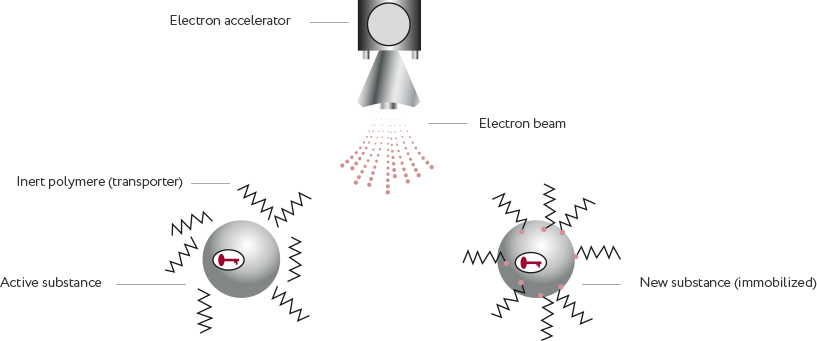
Immobilization of biologic active substances on water soluble electron-beam activated polymers.
Without chemical reactions and catalysis.
Electron beam as an instrument:

The drug has thrombolytic, anti-inflammatory and cardioprotective activity.
Thrombolitic mechanism is connected with direct destruction of fibrin strings, which form the main frame of thrombus. Anti-inflammatory mechanism is connected with influence on blood neutrophils and tissue macrophages oxidizing function. Cardioprotective mechanism is connected with myocarditis blood supply improvement. Trombovazim is a low-toxic preparation. Trombovazim® neither decreases platelet levels, nor influences coagulation time and bleeding duration.
Bioavailability of the drug when taken inside is 16-18 %. Maximum effect is observed 6 h. after intake. Systemic clearance is 1.2 ml/min, elimination rate constant with single intake is 0,057 min-1. It does not bind to blood plasma proteins and blood corpuscles. The preparation half-life at blood specific activities measurement is 12 minutes. It does not bind to plasma proteins and blood corpuscles. The drug has no cumulative effect if recommended doses and dosage frequency are observed. The preparation major pathway is renal (80 %). Partially metabolized and excreted by liver (20 %).
The drug should be taken orally 30-40 minutes before meals in the doses of 800-1600 U/day divided into two doses. Maximum daily dose is 2000 U. Course of treatment is 20 days. If no efficacy observed during the course of treatment, one should consult the doctor. Courses of retreatment can be given if necessary upon the doctor’s recommendation.
Heparin, dipiridamol, acetylsalicylic acid increase antithrombotic effect not increasing the risk of bleeding. Administration of the drug with antibiotics of tetracycline group (tetracycline, chlortetracycline, hydrochloride, oxytetracycline hydrochloride) increases effect of fibrinolytics. When necessary to use drugs containing bivalent metal salts (calcium, magnesium, zinc, iron) one should observe time interval of 40 minutes minimum between intake of Trombovazim and the drugs mentioned because of the possible enzyme activity reduction.
«Siberian Center of Pharmacology and Biotechnology»
CJSC, phone/fax: (383) 354-02-10
Legal address: 630090, RF. г. Novosibirsk, Academician Lavrentyev Av, 10.
Manufacture address: 630056, RF., Novosibirsk, Sofiyskaya Str., 20.